 |
 |
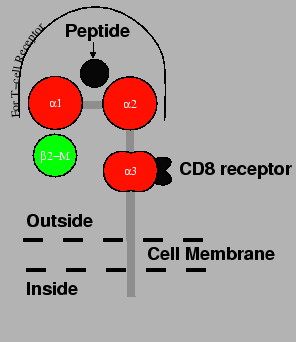
The class-I MHC molecules are termed HLA-A,HLA-B,HLA-C. There are also E, F, G and H, of which only A and B are thought to be important in transplantation.
MHC class-I molecules are found on the surfaces of virtually all cells.
The exceptions are the cornea and neurons. Some cells express alot more than
others. The are composed of a 45-kilodalton polymorphic MHC encoded heavy
chain, ![]() , and a 12-kilodalton monomorphic chain,
, and a 12-kilodalton monomorphic chain,
![]() -microglobulin. The polymorphic, MHC
-microglobulin. The polymorphic, MHC ![]() , chain is encoded on
chromosome 6 and the monomorphic
, chain is encoded on
chromosome 6 and the monomorphic ![]() -microglobulin encoded on
chromosome 15.
-microglobulin encoded on
chromosome 15.
The ![]() -microglobulin
component2binds non-covalently with the
-microglobulin
component2binds non-covalently with the ![]() chain and it is not attached to the
cell. It plays a role in the assembly and transportation of the
chain and it is not attached to the
cell. It plays a role in the assembly and transportation of the ![]() chain
to the cell surface.
chain
to the cell surface.
The heavy chain bears 3 major domains, each of these bears resemblance to the
Ig constant region and thus belong to the Ig superfamily. One of the domains
acts as a receptor for CD-8 (on cytotoxic T
cells) and the remaining two form a grove in which the antigen
to be processed is carried. If there is no peptide antigen in the groove then
the class-I molecule cannot be formed in a stable configuration. The amino
acids in the groove are highly variable which accounts for their ability of
these proteins to bind to many different antigens. There is room in the
groove for a peptide of about 9 amino acids. The hypothesis is that each
class-I allele may only bind a limited spectrum of peptides, all of which
are 9 amino acids long. The two ![]() subunits that form the groove for
the peptide, also act as the main site for binding by the T-cell receptor
complex.
subunits that form the groove for
the peptide, also act as the main site for binding by the T-cell receptor
complex.