



Next: Fixed acid:
Up: Normal acid base balance
Previous: Normal acid base balance
Index
Referring to equation 4 we
see that CO
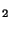 combines with water to form carbonic acid. The
carbonic acid then dissassociates into HCO
combines with water to form carbonic acid. The
carbonic acid then dissassociates into HCO
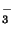 and [H
and [H
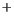 ].
About 15 thousand millemoles of CO
].
About 15 thousand millemoles of CO
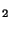 are produced per day.
This corresponds to 10 millemoles of CO
are produced per day.
This corresponds to 10 millemoles of CO
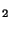 per minute. The
lung can remove CO
per minute. The
lung can remove CO
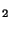 at a rate of 10-15 mmol per minute, so
that potential 10 millemoles of acid that could be created from
CO
at a rate of 10-15 mmol per minute, so
that potential 10 millemoles of acid that could be created from
CO
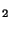 per minute are easily eliminated. In contrast the kidney
can only secrete 0.4 millemoles per minute of [H
per minute are easily eliminated. In contrast the kidney
can only secrete 0.4 millemoles per minute of [H
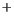 ] under
optimal conditions.
] under
optimal conditions.
Adrian P. Ireland
